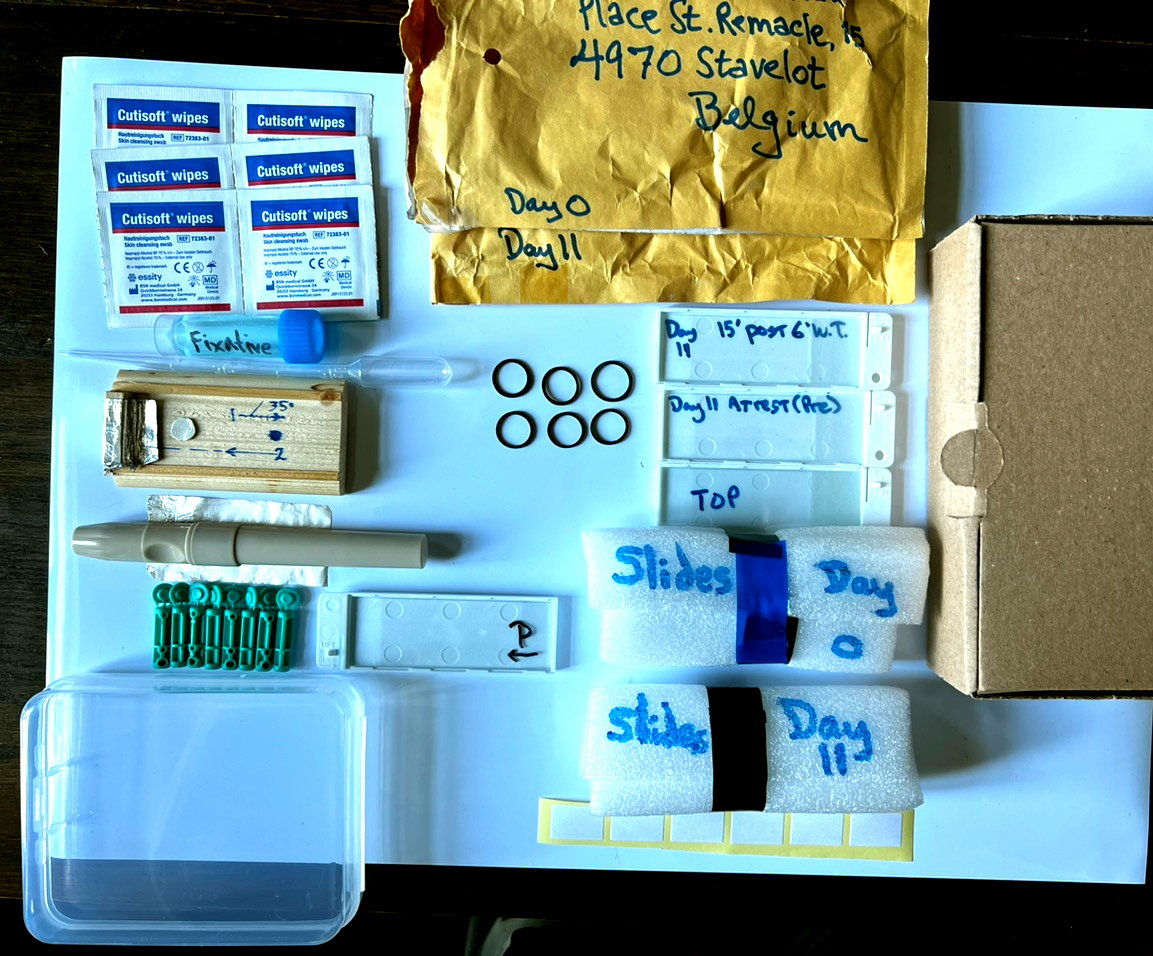
The Peripheral Smear Box and Contents
StudyLTCOVID.com
Thanks for visiting!
To translate this page, select your
language from the dropdown menu below:
Traduire avec le traducteur ci-dessus si besoin !
In the Big Box, you'll find the smaller
"Peripheral Smear Kit" box.
This box, close up ...
And in that box, is a smaller plastic box, ... like this below :
And in it and packed into the surrounding cardboard box is lots of stuff. I mean, equipment.
Now that it's unboxed, let's do a guided tour.
Left to right, top to bottom, clockwise.
Alcohol wipes. Used before poking a finger to clean and stimulate capillary flow in the periphery (i.e., get your finger ready to be poked).
Used after the poke to put a little pressure on the poke hole and seal it up. (Making it essentially invisible).
Addressed protective padded envelopes for sending back glass slides with peripheral smears. One for Day 0 (before intervention), and one for Day 11 (after intervention with light).
A 5 mL screwcap vial of fixative for peripheral smears, and a plastic pipette for drawing up some of the liquid. After air-drying the slide for an hour or so, the slide is placed in one of the plastic slide holders, and several drops applied to flood the area of the smear on the slide. After 10 minutes, this can be poured off. The rest will evaporate and disappear. Then the glass slide is placed in a slide holder before preparing it for its trip home.
In some countries, this liquid (buffered methanol) may generate problems at the Customs passage stage. So in such a case, it will be omitted, and slides are simply air-dried and sent.
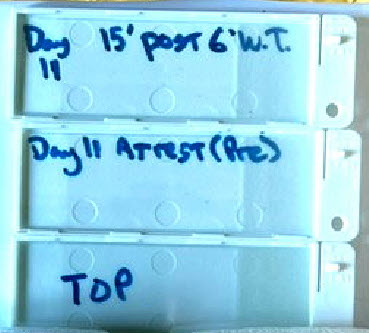
At left, the protective slide holders, just mentioned above.
Here we see them labelled for Day 11 samples. One holds a slide
that is the "Pre" Walking Test sample. The other, 15 minutes "Post"
the 6 Minute Walking Test (6'WT).
The one labelled "Top" has a slide placed in it in this image. Usually
it serves simply as a top cover, with no slide.
They slide together and clip shut, as demonstrated in the video
of the peripheral smear technique. Play with them. You'll get the hang of it.
If you are the point of fixing your slides that have thoroughly air-dried, here is a very brief video that presents how to do that.
Protective foam wraps, that the above protective slide holders are surrounded with. Here, seen held in place with some electrical tape. Several rubber bands would also work.
Do send back Day 0 slides when they're ready to go. Don't keep them around until Day 11 ! That's why there are two mailing envelopes as as shown above.
The typical mailing delay is bad enough. So send soon.
Again, Day 11 protective wrap.
Below the arrow, one sees 6 labels.
Labelling is of course very important to avoid sample confusion when processed in the lab. The current format for these labels is a bit smaller, since the previous larger ones interfered with slide storage. Initially, one can write with pencil on the ground-glass end piece of the slide. That's also the side for the sample. Add the label later.
Include:
Study ID#, Date, Day Number, "Pre" or "Post" 6'WT. There is room on the label if writing small with a ballpoint pen.
Slide holder platform. A dot of putty below the slide, keeps it stable. Rails on each side help stabilize as well. The written marks remind: size and position for the drop of blood sample. At #1 starts the process of backing up to pick up the sample with a second slide, letting it spread, before #2, pushing it rapidly towards the end block (covered with aluminum foil to keep it clean).
Several things at left. A piece of aluminum foil if needed to replace the one shown above. Lower right, the edge of a slide case, described above. A lancet device ("lancing aid") above, gets unscrewed to hold one of the 8 lancets shown below it. Use the device on #5 setting for an optimum finger "poke." Once done, use the lance end caps to cover the point and avoid injury before discarding.
These tiny rubber bands are included to serve as finger tourniquets. Place one or more on the selected "target" finger for a few moments before using the lancing aid and lance, and a good specimen should be generated. May be quite useful for anyone with the "Raynaud's phenomenon" seen in LTC to offset capillary constriction. (Remember to take them off eventually!)
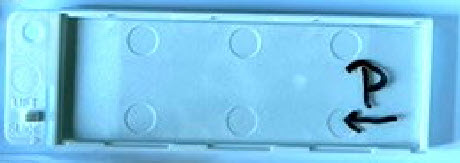
Finally, this shows what we call a "pusher slide" sitting in a protective case. It's labeled with a "P" to help destinguish it when unpacking your box. It's important characteristic is that its corners and edges have been ground smooth. That allows blood cells and plasma to flow along its edges and get smoothly distributed on the underlying glass slide. If used repeatedly, small clots will form along the edge of a "pusher slide," and it is no longer smooth. A streaky smear results. So after a use or two or three, moisten its edge with some water or alcohol and wipe the edge completely clean with a paper towel or Kleenex. In real laboratories, a good "pusher slide" is carefully cleaned after use and set aside for its next use on subsequent days. Lab techs have been known to fight over these.
So that's it ! Your "peripheral smear kit" has been unboxed.
To gain confidence in the technique required, practice it with some soy sauce or ketchup for
a time or six before poking your finger. Sending me a slide with dried ketchup on it may not
be that informative when stained.
The drop needs to be about 10 uL which you are not going to measure of course. The dot on the slide holder platform as shown above, is about the right size. Too big a drop is to be avoided. When looked at with a microscope, all the cells are clumped one on the other, and of little use for most of the observations being made.
A good smear when viewed with the microscopt starts off too thick (near the blood drop), ends up too thin, but has a "happy spot" where cells are nicely distributed one next to the other. This distribution is called "a monolayer." I could look at monolayers all day.
In fact, I have been looking at monolayers all day. Not everyone gets as excited about this.
If "Step 1" is rushed, the sample doesn't spread out enough along the "pusher slide." So a central long, skinny smear results that is limited in what it presents. So give it about 1 to 2 seconds to spread along the slide edge, then rapidly push in the other direction for "Step 2."
"Step 2" is pretty fast, usually less than a second. But don't break the slide!
Practice with the ketchup or soy sauce. Then take a break and make a burger.
Watching how its done in the video makes this much easier and stress free.
Again, fixation of an air-dried slide is covered here.
Soon, you may be looking for a job in a hematology lab.
As always, send along any questions: Questions@StudyLTCovid.com