Outreach
Survey on Duration of Illness
Embedded below is a 3 minute survey.
It's goal is to update previous data (see LTCOVID.com) for the duration of illness
associated with "long-term" COVID-19.
It includes estimates for those who are still suffering from symptoms and findings.
It also pursues an estimate of this length of illness for those who now feel that they
have recovered from "long-term" COVID-19.
Responding to this survey will provide you with an immediate response as it currently exists.
Here is a link to the survey to take it online.
Or copy and paste it to share with others as seen below:
https://www.surveymonkey.com/r/DURATION
You can respond to the survey below ...
Create your own user feedback survey
Let me be your guide
StudyLTCOVID.com
Thanks for visiting!
To translate this page, select your
language from the dropdown menu below:
A good guide has many times walked the path you now find yourself on.
Outreach includes saying at times; "Show me the way."
Moving towards an understanding of the "Long-term" COVID-19 situation includes a map that the guide always carries during the walk. I chose to write "situation" and not "illness" because "Long-term" COVID-19 has already moved from simple involvement at that personal medical level of being sick, to widespread effects in world healthcare reform, labor markets and economies.
That map is not yet completely drawn in, since many parts are still missing.
It leaves out certain inhabitants of this environment who are also involved and clearly present.
But much is known, can be shared, and the sketched map gives us the terrain.
It shouldn't take long to know if one is at the beach, or in the mountains, unless you are blindfolded.
Other senses will of course reach out continuously to suggest where you are.
Those are our instincts. They also exist and with time become more developed in scientific research. One learns to observe with all tools available.
Unfortunately, many who arrive for this trek arrive at the start, still blindfolded.
So first, let's remove our blindfolds and have a look at what that "average person" with "Long-term" COVID-19 can teach us. Before picking up rucksack and water bottles, this walk begins by identifying the home point on the map. It's the place to always know the path back to.
Just like when you turn the key and push the door open where you live: "Yes, this is home," is what you say with your entire being. Feeling protected.
Not everyone's home is the same. Especially if one extends that globally from apartments and estates to huts. Similarly not everyone with "Long-term" COVID-19 will live the same experience.
Yet most will easily find themselves in the following summary of average findings.
As you prepare to view the video below, a word about the numbers.
Here in Belgium, the decimal separator is a comma (",") and not a period (".").
So when you see "1,234" that is not "one thousand, two hundred thirty-four."
It's "one point two, three four."
Now have a look.
Once one begins to feel some mastery of the terrain, keeping this basic map presented above close at hand, one can next begin to ask further questions about those parts of the map that are missing.
Answers obtained will give direction to further exploration. Done of course safely, and without
becoming lost in the darkness of unknowing or finding oneself at a dead end.
But if unfortunately at a dead end, where is the path back to home?
Many of those questions and their answers will be presented subsequently, adding a
deeper understanding of the terrain of "Long-term" COVID-19.
These should always have a traceable path back to the home point on the map as just presented above.
For convenience, here is a link to the this video of our starting point, that you can take with you as you proceed:
Here at StudyLTCOVID.com, our present work always remembers this home point.
Enrollee tasks and what these require
StudyLTCOVID.com
Thanks for visiting!
To translate this page, select your
language from the dropdown menu below:
Here is an assembled list of what enrollees will carry out.
Some will decide to eschew participation because what follows seems extensive.
Others will understand that these required tasks represent a process that should clarify if a specific intervention with light is of any use to those suffering "long-term" COVID-19, or not.
Part of the work is carrying out the intervention with a specific light source.
The other part is gathering and transmitting the data to allow analysis of potential effectiveness or lack of benefit. Clearly important: no data, no results, no analysis, no meaningful answer.
-
- Participation requires committing to 31 days in sequence of interventions and measurements once begun. One cannot take a week off then come back to it. (If that already seems rather long, the last 10 days are "days off" or rest days. So 21 sequential days of intervention and study).
- "The intervention" is guided by a process of randomization of enrollees. Not all will be doing the same thing. Nevertheless each arm of the study (EA, 20 individuals) and (NEA, 20 individuals) will carry out identical components as follows:
-
-
- "Intervention group": 10 minutes of structured exposure to light passed over the head region. (10 individuals in each of the two arms).
- "Intervention + group": an additional 10 minutes of structured exposure to light over the posterior thorax (lower lung region in back). (10 individuals in each of the two arms). So a total of 20 minutes of intervention each day for those randomized to this group.
-
-
- Data gathering and reporting
- There are 4 "Full Study" days (see Participant's Calendar). These include:
- On Day 1, 11, 21, 31 -
- Responding to an online questionnaire about present symptoms and severity
- Responding to an online mental status exam aimed at assessing cognitive function on each of these more complete study days
- Carrying out an assessment of visual acuity, recording and transmitting results
- Using an innovative (and essentally painless) device to obtain a small blood sample for use in laboratory analysis of markers of inflammation and cellular function
- Measuring vital signs in a protocol designed to assess impact of a very light physical effort, specifically:
- Measuring after 5 minutes at rest - temperature (°C), peripheral oxygen saturation (spO2), Systolic and Diastolic blood pressure (mmHg), Heart Rate (bpm). These masurements are made with supplied equipment, and results recorded and transmitted to the study centre.
- A 6 minute period of specific physical effort.
- A recovery period to assess effects of the brief physical effort, with the same measurements made and recorded at 0, 5, 10 and 15 minutes after the physical effort.
- There are two sequential 10 day periods of intervention with light
- Day 1 to 10
- After 5 measured minutes of rest, the same vital signs listed above are measured.
- Intervention with "Light #1":
-
- For some, this light will supply a placebo or sham wavelength of red light. This is decided by randomization. The actual intervention light follows below.
- For some, 10 minutes of structured exposure to light passed over the head region.
- For others, an additional 10 minutes of structured exposure to light over the posterior thorax (lower lung region in back).
-
- Day 11 to 20
- After 5 measured minutes of rest, the same vital signs listed above are measured.
- Intervention with "Light #2":
- Each enrollee has been presented with 1 lamp holder and two LED light sources. On Day 11, the light initially in the holder ("Light #1") is exchanged for Light #2. Day 11 is referred to as the Crossover Day. If the initial light source had provided a placebo or sham wavelength of light, Light #2 will now be the intervention light with effects under study (and vice versa). Some will be switching (in a blinded and randomized way) to the placebo device. It is important and reassuring to note that ALL enrollees will have access at some point during this study, to the device presumed to be the active intervention.
- For some (selected randomly), 10 minutes of structured exposure to light passed over the head region.
- For others (selected randomly), an additional 10 minutes of structured exposure to light over the posterior thorax (lower lung region in back).
- Day 21 to 30
- These are easy days! They are days off, or days of rest. They are formally included in the protocol to provide a period without any intervention. They are followed by Day 31 which is the final "Full Study" Day.
- No interventions with light
- No vital signs to take. (One is free to do so if desired, but no data need be sent in).
- No other tests or questionnaires.
- Enrollees are free to carry out these days and activities ad lib, as desired. But Day 31 must not be forgotten! It is the final "Full Study" Day used to assess persistance of effects, or lack thereof.
- These are easy days! They are days off, or days of rest. They are formally included in the protocol to provide a period without any intervention. They are followed by Day 31 which is the final "Full Study" Day.
- Day 1 to 10
- On Day 1, 11, 21, 31 -
- There are 4 "Full Study" days (see Participant's Calendar). These include:
Support for Enrollees
All of the above tasks require the equivalent of a "User's Manual" to accomplish the present study with success.
-
- This site is that "User's Manual" - if questions arise that have not been addressed here, every effort will be made to remedy that promptly. Write freely to "Questions@StudyLTCovid.com" for instructions or support.
- To obtain uniformity of measurements made, the required equipment is supplied and explained through Demonstration Videos on this site. These have been structured to include much detail. Some will say "too much detail," but the goal is to be complete and not leave anyone feeling lost with unanswered questions. Scientifically, this should improve results by reducing variability in the measurements carried out by individuals in various locations and not in a laboratory or hospital clinic.
- If no results transmitted: no data: no study. So facilitating this part of the process is essential to protocol success. An individual's results will be sent to specific email addresses. Results are then protected as they arrive, through addition to several databases. All such transmissions respect personal privacy, belong to the present study, and will not be shared in any other way. At the end of the study, results will be presented on this site, and again, without identifying any individual personally.
- Enrolling in the study also involves providing informed consent. This exists to protect enrollees and assure a minimum of risk and a maximum of benefit. It protects the study in that it contributes to trustworthy results. That Informed Consent Process will be presented elsewhere on this site.
- Care has been used to maintain "double-blinding" of study participants including the Principle Investigator. If questions arise, no one will know until after the study has run to completion, whether an intervention or sham light source is being used, for example. Incoming results will not identify until the end of the study, the "arm" and "group" tied to the data. An enrollee must always include her or his "Study ID#" with any data transmmission. Absent that vital identifier, data processing becomes uncertain if not impossible. No further information is provided to avoid countering the study's required blinding and controls.
- While keeping to this "double-blind" state, daily online scheduled meetings (using Zoom) will be offered to enrollees to answer questions and address potential problems if any are discovered. Any research project can be expected to include unanticipated unknowns and surprises. Hopefully, these will be minimal if our pre-study work has been well done.
The components of the present study have been organized into a 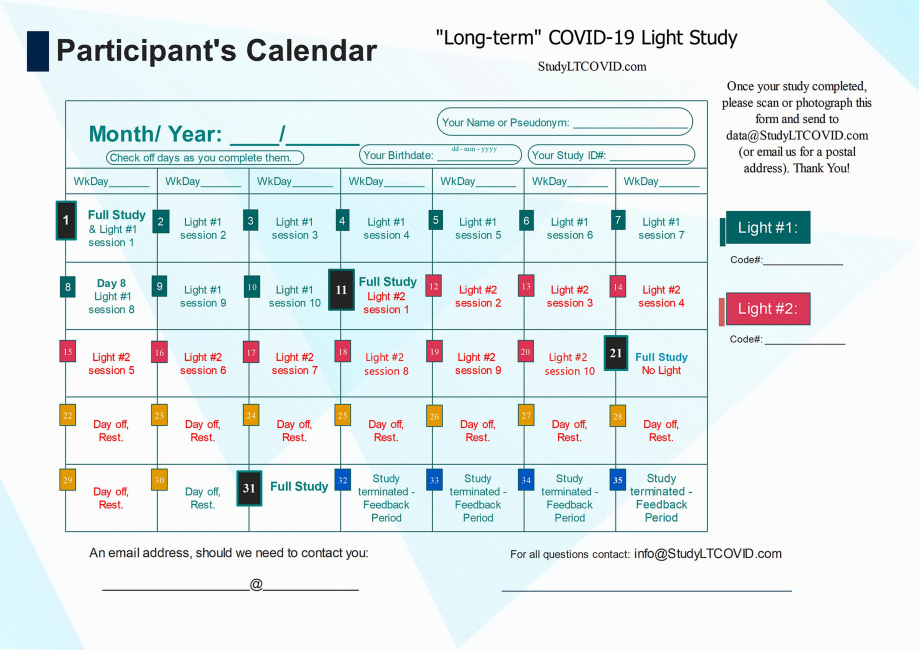
- Enrollees use the above Calendar to check off completed days and see where they are in the study. This for their own use. No need to send it back. (Perhaps a picture or scanned copy would be nice at the end, on Day 31).
- To be reminded of switching lights used on the "Crossover Day," Day 11.
- To get used to their assigned Study ID#____. It is used for transmitting results of vital signs, visual test and other interventions. It is the only identifier used as well to protect enrollee privacy and the randomization process.
Details of What-Happens-When, are provided for the Participant's Calendar.
Most importantly, it serves to crunch what seems like a lengthy Things-To-Do list as presented above, into smaller, daily bite-sized morcels.
Enrollees participate each day in this study, and for which we are grateful.
Enrollees also have a life.
So the study has been structured to minimize interference in an enrollee's day.
<<<< Previous Page (Criteria for Inclusion)
List of 20 most frequent symptoms
StudyLTCOVID.com
Thanks for visiting!
To translate this page, select your
language from the dropdown menu below:
The list is based on responses from our prior questionnaire last Fall, 2020.
It is used for inclusion/ exclusion of a potential enrollee in the study.
It is used for subjective assessment of effectiveness of an intervention with light,
at 4 specific times during the study (see Participant's Calendar).
Those enrolled in the study have experienced at least 10 of the following at some point during their involvement with "long-term" COVID-19. They continue to experience at least 5 of these findings at time of enrollment in the study.
These items are presented in order by frequency of response from the prior questionnaire.
Difficulty Concentrating
Marked Fatigue
Slowed Thought Process
Frustration/ Impatience
Anxiety
Tearfulness
Headaches
Simple Things Are Too Complicated
Muscular Aches & Pains
Depression/ Sadness
Diffuse pains: head, chest, abdomen, back, extremities
Fearfulness
Cardiovascular symptoms (abnormal heart rhythms, palpitations, high or low blood pressure, very fast or slow pulse, swollen extrelities, blood clots, blue extremities, intolerance for physical exertion with shortness of breath).
Shortness of breath
Memory Problems
Joint Pains, Problems Moving
Dizziness
Feeling like an Outcast, a Pariah
Intermittent Tingling in Fingers and Toes
Panic
The above list is offered here as a checklist in PDF format.
<<<< Inclusion/ Exclusion criteria, explained
<<<< Home
Criteria for Inclusion in This Study
StudyLTCOVID.com
Thanks for visiting!
To translate this page, select your
language from the dropdown menu below:
There exists a great variablility, a wide spectrum, of what those with "long-term" COVID-19 experience on most days.
In very general terms, an individual became ill, and that illness has now lasted longer than expected. But to help people decide whether to seek enrollment in this study or not, the following will be of use. Almost no individual with this illness has experienced all that is presented below.
As you read on, and if a potential enrollee because you have "long term" COVID-19, see how much this sounds like your experience.
Our guide in this is our questionnaire responded to from last September to November of 2020.
This, fully reported at LTCOVID.com.
In many ways, responses obtained therein helped to define the natural history of this illness, or at least, as it presented at that point in time. Nothing says that new symptoms or a change in level of importance, can't manifest somewhat differently since then. At that time, many had first become ill in March of 2020, and something about the virus at that time, (its genetic makeup for example), may now have changed. The effect of vaccination for this viral illness was at that time hoped for but absent. Now it has become a reality.
Since our prior work, other studies have suggested that the entity of "long-term" COVID-19 continues, independent of vaccination, and remains a specific and definable entity. Before deciding to group it with other illnesses felt to be similar, here we will carefully maintain focus on "long-term" COVID-19 and this study's specific findings.
We are enrolling 40 individuals in this present study.
Twenty (20) have a pre-illness record of significant frequent athletic activity. They are the "endurance athletes" arm of the study, abbreviated EA. They will help to demonstrate the important impact of "long-term" COVID-19 on even intact or "in shape" bodies. This is not an illness limited to those who were "out of shape" when becoming infected.
Twenty (20) others are suffering with "long-term" COVID-19, but are not athletes. They can in fact not be athletic at all. They are the present embodiment of those respondents surveyed last Fall. They are titled the "Not endurance-athletes" abbreviated NEA to identify those enrolled in this arm of the present study. Body composition data were obtained as part of the previous questionnaire.
While not used in the present study to set specific limits, these data give a sense of physical size that is of use in trying to match these "Not endurance-athletes" for the present study.
A simple summary is that marked overweight or underweight was associated with more problems when iinfected with SARS-CoV-2. Most respondents last Fall were overweight by established standards. More on this below.
GENDER
A majority in each arm of the study are female. Prior study: 90.37% women; 9.63% men.
So in each arm of this study of 20 individuals, that would give 18 women and 2 men. To help distinguish gender differences if they appear, we shift this to 15 women and 5 men in each study arm. That's a total of 30 women and 10 men studied. One aim of this shift is to identify gender differences in response to the intervention, if present.
We will present a tally of enrollee gender on this site, so outreach to those still needed can be carried out. Of course as we begin, that tally is 0 women and 0 men enrolled! That will look like this next line, and will be updated here as enrollment proceeds.
June 5, 2021: 0/30 women & 0/10 men.
RACE
Respondents to the aforementioned questionnaire were white 89% of the time.
Using group comparisons, some significant differences were previously identified linked to race.
These have been presented halfway down on this page.
While of interest, their careful definition is not possible in a study of the present size.
While enrollee race will be noted, no specific targets based on race will be used in approaching enrollees for this study.
AGE
Mean age for questionnaire respondents was 48.19±10.90 years.
Two standard deviations to either side gives a range of 26.4 to 69.9 years.
While this adds focus to the process of selecting enrollees, deciding to use "48 years old" as a criterion is not helpful. This may be expecially true for the endurance athlete arm.
Simply put, there are no age limitations for this present study.
BODY SIZE
Most in our prior survey were overweight. They also tended to be slightly taller than average.
While some were obese and others underweight, as mentioned both extremes can pose problems for those with COVID-19 infections, and be associated with a poor outcome.
In this present study, Body Mass Index, though calculated and presented for subsequent analysis, will not be used to include nor exclude potential enrollees.
The study does involve 4 periods on separate days of 6 minutes of very mild exercise (marching at a specific pace), and detailed elsewhere. If one's body size precludes even this usually unstrenuous effort, one should not seek enrollment. Being affected by "long-term" COVID-19 may change a "usually unstrenuous effort," to something quite challenging. This, even for previous endurance athletes. Each individual should give this careful consideration.
PRE-ILLNESS SOCIAL ROLE
This refers to married or single, breadwinner or homemaker, parent or not, and specific employment. These were discovered and presented here.
No specific targets based on pre-ilness social role are used in this study.
SMOKING HISTORY
While it seems evident that fewer smokers will be found in the endurance athlete arm of the study, 57% were non-smokers who responded last Fall, leaving a number of smokers greater than the 13% to 14% found in the populations concerned.
While not specifically seeking to enroll smokers only, smoking should not be taken as a reason for exclusion from this study.
MEDICATIONS & THERAPIES
Use of presecribed medications, nutritional supplements or other (non-allopathic) therapies are not used as a criterion for inclusion or exclusion from this study.
ILLNESS SEVERITY
Respondents characterized their illness as "Moderate," in 82.35%. This is an illness in which the person stayed home but became very sick, had many symptoms, and that illness lasted longer than anticipated.
Most (68.2%) respondents last Fall had not been hospitalized with this illness. 8.89% one day or less, and 9.63% more than one day.
Prior hospitalization is not an inclusion or exclusion criterion. It is assumed that anyone still in hospital will not be seeking enrollment. If the illness required recent hospitalization (in the last month for example), continuing convalescence and recovery takes precedence on participation in this study. This is especially true since the study includes 4 sessions of 6 minutes of exercise.
DIAGNOSIS of COVID-19
Prior respondents lacked a medical diagnosis in 28.89%. Yet they responded to the questionnaire as persons affected with "long-term" COVID-19. This is of course a critical component of how patients with this illness present to the extant medical system. This has been covered here in the Answers section, Question 20. Diagnostic tests were negative in 38.93%, which was a majority of respondents. Question 26 details other responses given. Only 21.37% reported a positive nasal swab. When asked phrased in reverse, 56.91% had a negative nasal swab, 37.4% a negative oral swab, and 27.64% a negative antibody test.
While a positive or confirmatory test for an illness is always comforting from a diagnostic perspective (not necessarily comforting for the patient), should only those with a positive antigen or antibody test be included in this study?
We think not, preferring to rely on the pattern of physical and emotional symptoms as presented below to decide on study admission of a potential participant.
PRE-ILLNESS HEALTH STATUS
Self-assessment of health was presented in Question 31's answers.
The majority response was "Status typical for age; Ok I guess' (42.22%). The second most common response was 'Perfect health' (23.70%). "1 important health problem" was present in 21.48% of respondents.
Symptoms commonly experienced before this viral illness have been presented in the Answers to Quesion 37 of the prior questionnaire. The most common frequent prior symptom was 'Headache'. The list is long, but allows consideration of chronicity of some symptoms also attributed to the "long-term" COVID-19 illness.
Barring severe ongoing medical illness, complaints as identified in Question 37 need not exclude a person seeking to participate in the study. Cardiovascular system involvement was present in 17.56% before COVID-19. In interviewing a potential enrollee, such symptoms that suggest possible increases in risk of further injury or illness from present study participation, will be carefully sought out.
Prior symptoms before acquiring COVID-19 or their absence, are not used to exclude nor include a potential participant.
SYMPTOMS
This study includes evaluation of the effectiveness of an intervention in the setting of "long-term" COVID-19.
This evaluation takes the form of a questionnaire repeated at 4 times, and focusing on levels of severity of specific physical and emotional symptoms and any possible evolution in these during the present study. This represents a subjective assessment. Objective data are also gathered separately as noted elsewhere.
This list is based on the obtained frequencies of those symptoms discovered by the questionnaire last Fall. The top 20 symptoms, sorted by frequency are further pursued in the present study.
It would add little to the study if an enrollee had none of these symptoms, even if considering herself or himself a victim of "long-term" COVID-19.
So an inclusion/ exclusion criterion has been established as follows:
In reviewing this list of 20 most frequent symptoms, a potential enrollee in the study must have experienced at least 10 of these at some point, and still be experiencing at least 5 currently.
On the page linked to above, the list is also offered as a checklist in PDF format, but also found here.
Other reasons for inclusion or exclusion are related to the demands of participation.
What is expected of a participating enrollee?
One may fit the mold of someone who should or could be in the present study.
And yet, what is asked for and expected, may not suit a given individual.
The study requires daily motivation and activities for 31 consecutive days.
Not your style? ...
This becomes a self-imposed reason for exclusion. And no hard feelings!
If you know a person who you think is right for this study, don't hesitate to copy and share the
link to this site and this page. (Right click on the link and select 'Copy link address' and paste in a message or email).
These tasks are defined by the study's protocol which can be found here.
These enrollee tasks and what they require will be presented next.
Enrollee Tasks >>>>
<<<< Previous page
<<<< Home
