The Participant's Calendar
StudyLTCOVID.com
Thanks for visiting!
To translate this page, select your
language from the dropdown menu below:
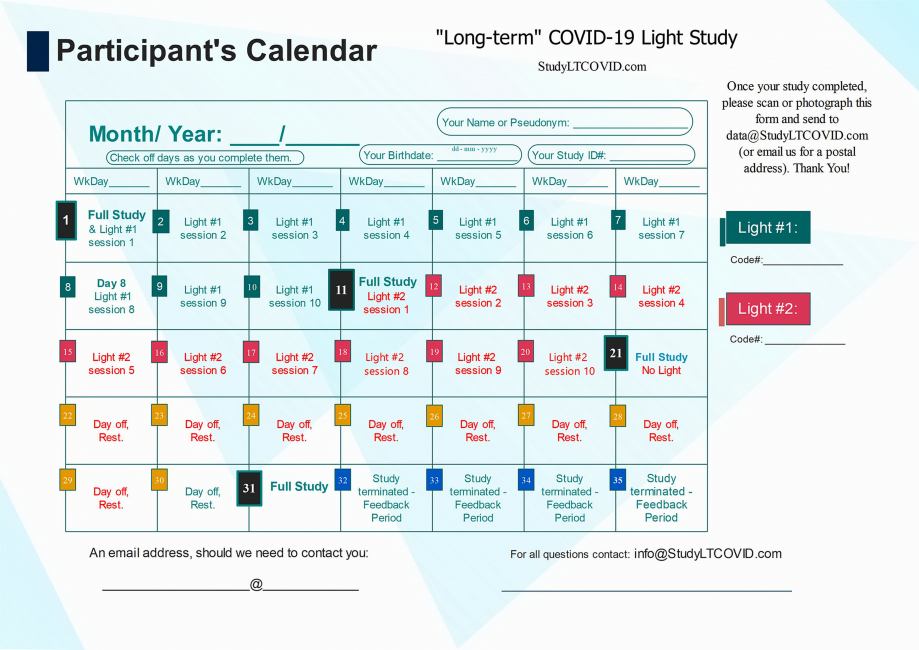
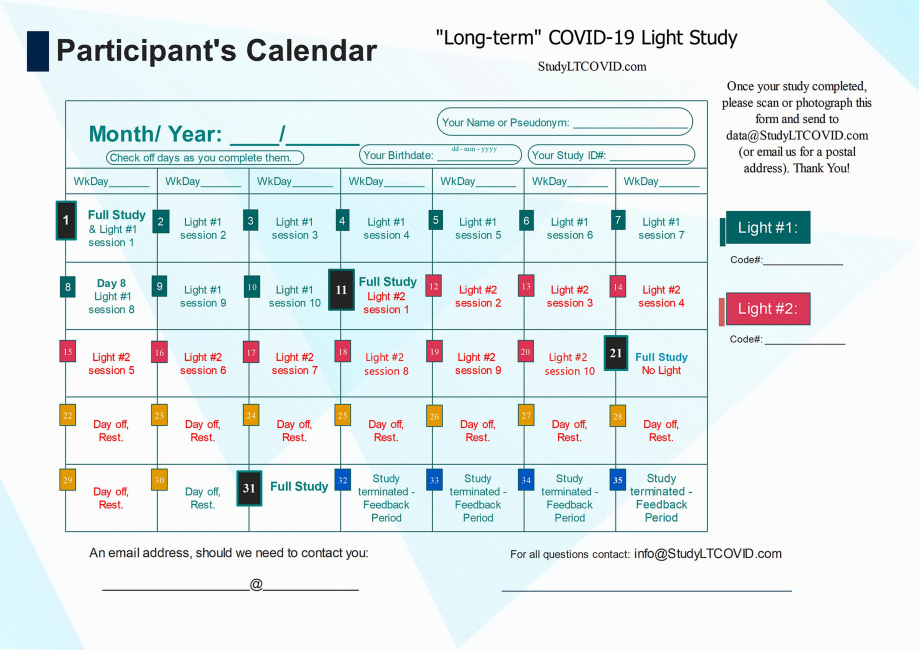
This is the calendar that allows each participant to find more easily, where they are in the study.
The image above is a bit hard to read for detail, but here is the calendar in PDF which you can enlarge as needed to read more easily.
Let's look at some details of this protocol lasting 31 days:
One notices:
-
-
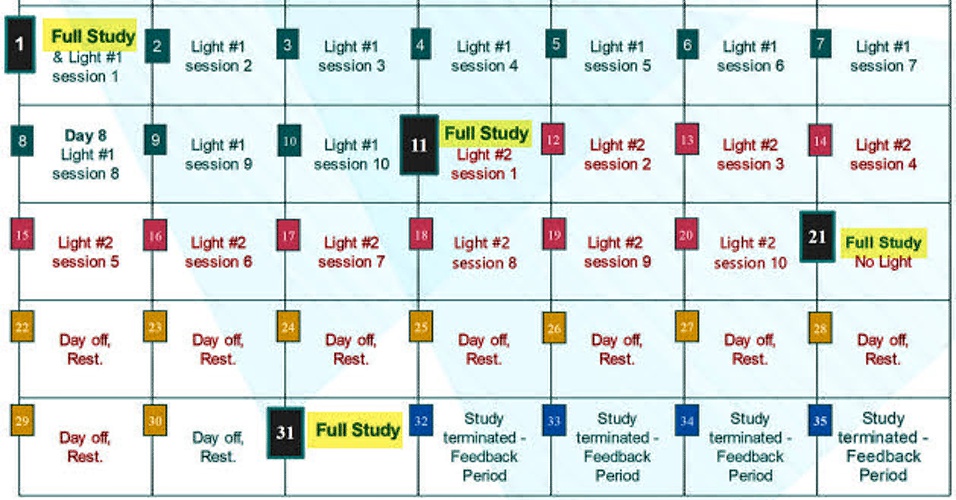
- 4 "Full Study" days: Day 1, 11, 21, 31
- 10 "Light #1" days on days 1 thru 10
- 10 "Light #2" days on days 11 thru 20
- 10 "Day off/ Rest" days on days 21 thru 30
-
Things to emphasize:
-
-
- Day 11 is referred to as the "Crossover Day." - participants in the study each receive two different LED sources that emit light, here simply listed as "Light #1" & "Light #2." Each participant is told from the start, which light to begin with. This choice has been randomized, but eventually, all participants use both lights for 10 days, one after the other. Staying to the order assigned is essential. Otherwise, things will get very confusing! ("I liked the other light better. I think I'll switch back..." DON'T DO THAT!!). Each device has a Code# marked on it. As shown on the right in the top calendar on this page, these numbers are also identified on the enrollee's calendar as "Light #1" and "Light #2."
- "Full Study" days are busy days. The following are accomplished:
-
-
- A Questionnaire about symptoms present on that day and level of symptom severity.
- A Questionnaire that is a very quick Mental Status Test, which is timed from start to finish with the provided chronometer. Duration of the test is an important data point. But this is not a race. Since "brain fog" is a common symptom of "long-term" COVID-19, this short test helps to quickly and objectively define how things are going with "brain fog" and other aspects of brain executive functioning. Are these getting better during the study? Each participant's data will tell us once the study has been completed.
- A Visual Acuity test using the 10 foot Snellen eye chart, and associated material supplied, and as described here in a video. The rational for including this in this study has been described.
- After a measured 5 minute period of rest, Vital Signs are obtained with the instruments provided. These are not "high tech" medical measurments like an ECG machine or CAT Scanner. But for those who have lived for years professionally in contact with patients being cared for, a patient's vital signs come to merit great respect. They are often the only indicator that something has changed in a person's physiologic status. "Vital Signs" in the present study always include:
- Oral Temperature in degrees Centigrade, taken with an infrared thermometer. We have all recently seen the images of their use in public, usually aimed at the forehead, since masks are often in place. Our preliminary studies have made it clear that aiming into an open mouth provides more useful results, especially when small changes need to be identified and confirmed. These provide results very quickly.
- Percent Oxygen Saturation measured with a pulse oximeter. Those we have provided are shown in a video of these measurements. One push on their button turns them on. A second quick push inverts the numbers to make them easier to read if needed. They turn off automatically after use.
- Blood Pressure (systolic & diastolic) & Heart Rate. These are measured with a provided device placed at the wrist. These devices are quite sensitive to position of the wrist above or below heart level. So to assure reproducible measurements, the correct position is defined and used for all measurements. When used with the provided support, values have been compared with a professional device and found to have only a 1% to 1.5% error, when comparing calculated mean arterial pressure. The manufacturer suggests a ±3% error. We found them even more accurate than that. A very detailed video presents these here.
- "The correct position" is assured by using the wooden Vital Signs Support device made for this purpose for our participants. Exact positioning when using them is presented in the above video.
- Next, a Walking Test is carried out for 6 minutes. This is shown in a video. This has been carefully constructed to be not excessively strenuous. An endurance athlete might be tempted to walk this test backwards since it is so easy! Nevertheless, anyone experiencing discomfort during this usually easy challenge, should drop out, measure Vital Signs at that time, and not resume this Walking Test on the same day. If one doesn't have the room, just marching in place should be adequate, as long as done in time with the drum. To help keep the pace a CD is included with the other devices, and an MP3 can be downloaded here for use on a computer or other device. You can email it to yourself and listen on your smartphone as you gad about! Keep the beat and follow the drum so your exercise routine matches that of other participants. And be ready to take vital signs immediately after exercise.
- Vital Signs are repeated to assess rate of Recovery, after the walking test. Normally, recovery should follow within 5 or 10 minutes after stopping this easy exercise. This may not be true for a person with "long-term" COVID-19. Obtaining these rate of recovery data are what we are after with these repeated Vital Signs. Chronometer(s) are provided and described, to keep measurements on time. It's easy to get behind!
- At 0 minutes - this as soon as practical after the end of the Walking Test. We have found that keeping the wrist BP device and pulse oximeter in place while walking, does not get in the way. It makes for a speedier return to the correct position on the Vital Signs Support and simplifies '0 minute' mesurements. The first thing to do at 0 minutes is to start the chronometer (stopwatch or countdown timer) so that subsequent measurements are done right on time. Five minutes comes up sooner than expected when one is still getting the hang of making these measurements.
- At 5 minutes after end of the Walking Test, Vital Signs are repeated. Again these always include: Temp in °C, Oxygen saturation (%), Systolic and Diastolic Blood Pressure (mmHg), and Heart Rate (bpm = beats per minute).
- At 10 minutes after the end of exercise, and as reminded by watching the chronometer. Mention is made here that results are noted as they are obtained on the supplied sheet covered with its plastic jacket, with a supplied 'White Board' erasable marker. Easy to take a picture of to send in to Study Central.
- At 15 minutes after exercise, Vital Signs to assess rate of Recovery are obtained a last time, and results noted. These are subsequently sent to the study center to avoid data loss, and as described elsewhere. Vitals@StudyLTCovid.com
- A clarification: In several spots in the recordings, the recovery data times are given as 5 and 10 minutes. We added the 15 minute data point after creating these recordings. This based on results from a small control group. The 15 minute measurement should not be omitted.
- A blood sample is obtained as described on the linked page. This does not require the usual medical equipment of tourniquet, needles and syringes, blood tubes and a trip to the lab! Instead, a very simple and essentially painless "finger-stick" type device (as used daily by most diabetics), is used for sampling. Details of this process, which aims to look at levels of several markers of inflammation in the blood of those with "long-term" COVID-19, are described separately. One can be squeamish about this finger pokes. They are really quite bearable, and the blood tests are important. It's only 4 times.
-
-
-
- All of the above represent an assessment of how the person with "long-term" COVID-19 is doing during the present study. This assumes that more is being done than simply measuring data over time. An intervention or its control (placebo) is also taking place. The ultimate goal is to use the above tests to define the effectiveness of this intervention, which makes use of specific wavelengths of light. So in addition to measurements and samplings on "Full Study" days, each enrollee carries out that intervantion. In prior published studies this has variously been described using the terms: photobiomodulation (PBM) or low level light therapy (LLLT). Details about its use for many other interventions besides "long-term" COVID-19 have been published and are linked to here. And a review in the setting of COVID-19, here.
-
-
-
-
- That intervention with light lasts 10 minutes for most participants (half).
- Some individuals have been randomized to an additional 10 minutes of light and as specifically defined here. You will be told ahead of time if you have been selected to be in this subgroup or not. Nothing about you or your situation decides this assignment. It has been decided by the randomization process. If you cannot participate in that additional 10 minutes of intervention with light (not more blood tests!), let us know.
- So "Full Study" days always end with the interventions using light.
- The 10 minute intervention is presented in detail on this page.
- The additional 10 minutes for a total of 20 minutes process is found here.
-
-
-
TO BE CLEAR:
If this study confirms objectively that this intervention with light is effective for those with "long-term" COVID-19, know that ALL Participants In The Study will have received this intervention during the study. That is why two light sources are sent to each participant. One may in fact be using the "sham" light first. But after the "Crossover Day," you will switch to the true intervention device. Of course, you may have been randomized to start with the "real" device and eventually switch to the "sham" device. What should declare that loud and clear is not the participant, but the participant's results at the end of the study. Guessing which is which serves no purpose. Switching from one light to another by personal whim is not only counterproductive, it destroys the study. It makes all the work involved by all participants, pointless. Even worse: it may suggest something that isn't actually happening.
To do a good study, knowing which light is being used should not be too closely pursued.
And at the study center, we don't want to know which is being used either. Not until the study has been completed. In study terms that's referred to as "blinding" and is a critical component of doing a good study of this nature.
Therefore from the start, a participant may not notice any change whatsoever. But if these first days seem a rather bland experience, don't use that as a reason to quit after a few days! Keep up the effort!
We are quite aware that asking a person to do all of the above work is quite a task. We would not permit ourselves to impose this on a person, knowing that all days of intervention are with a "sham" device only. That seems to us, quite unfair. So know that all participants will receive the intervention that we hope will do quite a bit of good to someone still suffering with "long-term" COVID-19. Eventually, your carefully collected data will tell!
--------
The other days that are not "Full Study" Days represent the majority of days on the Participant's Calendar as seen below.
-
-
- Here is how Days 2 to 10, and Days 12 to 20 are carried out:
- One prepares the instruments for taking Vital Signs
- A measured 5 minute period of rest while seated with the Vital Signs Support, in the same place as on "Full Study" days to keep conditions the same, starts the process.
- Vital Signs (Temp, spO2, BPs, BPd, HR) are measured and results noted.
- Vital Signs results are prepared and sent to the study center as described.
- The light intervention is carried out:
- 10 minutes for most
- 10 + 10 minutes for some.
- On these days:
- No eye test
- No questionnaires
- No finger-stick for blood tests
- No Walking Test
- No repeat Vital Signs at 0, 5, 10, 15 minutes.
- Here is how Days 2 to 10, and Days 12 to 20 are carried out:
-
If the Participant's Calendar and the events it schedules leaves you with questions, don't hesitate to send them our way!
Questions@StudyLTCovid.com
If you have thought of something we hadn't thought of, ... all the better!
As the study begins, issues of putting this all into practice have been addressed on the LTCOVID.com site. This might seem an appropriate time to review these.
Does this full protocol summary just seem like "too much" for you right now.
Perhaps you'd be a good candidate for our 10 day "Quick Impressions" subgroup.
Have a look at that link.
If your ready to go as a participant, have a look at the basic information we're asking you to provide. The goal is to make the study safe for you, and meaningful and manageable for us.